
What readers will learn in this article.
- How pressure and temperature changes cause cavitation.
- How cavitation causes damage to equipment.
- Gas entrainment compounds the problem.
- Methods to reduce the occurrence of cavitation.
What is Cavitation?
Cavitation is the occurrence of vapour bubbles in a liquid. A vapour bubble will form when the pressure in a liquid falls so low it boils or the temperature rises so high it boils.
Vapour bubbles can only occur if pressures are sufficiently low or temperatures sufficiently high. Until these conditions are reached there is only liquid and when exceeded there is only vapour. Water will boil at 100 oC (212 oF) at sea level pressure of one atmosphere or 101kPa (14.7 psi). Drop the pressure to 50% of an atmosphere by creating a partial vacuum and it will boil at 82 oC (180 oF). Drop it to 2% of an atmosphere (98% vacuum) and the water boils at 18 oC (64 oF). Liquids boil at temperatures distinct to themselves.
If you look at a saucepan of boiling water on the stove you will see vapour bubbles rising from the bottom of the pan and exploding at the surface. They start small at nucleation sites on the saucepan floor and grow bigger as the pressure of liquid overhead decreases during their rise. The saucepan is cavitating right in the kitchen. We call it boiling. The same situation develops in a cavitating pump.
Cavitation can occur anywhere that the right low pressure conditions develop. Examples are downstream of control valves as the liquid ejects out, the tips of propellers and agitators moving at high speed, downstream side of instrument probes in pipes containing fast moving liquid and pump impellers.
Cavitation becomes a problem when the bubbles cause damage to internal parts from implosions as the pressure again increases or when the vapour bubbles block off the available liquid flow area and reduce capacity.
What Causes the Pressure to Drop so Low?
Bernoulli’s Law states that the higher the speed of a moving liquid, or gas, the lower the pressure. A pump moves large volumes of liquid quickly from the suction inlet to the discharge outlet. If the liquid’s speed through the pump becomes fast enough to create sufficiently low-pressure conditions it will boil off the liquid and form vapour bubbles.
But the trouble starts even before we get to the pump. Each inch and millimeter of suction pipe, each elbow, every valve, each strainer, all protrusions and surface roughness, any change of flow direction before the pump rob it of pressure. Friction loss robs the liquid of energy. Even worse is when the pump has to suck liquid from a location at lower pressure or at a lower level than itself. For the liquid to move to the pump, the pump has to make a vacuum.
The pressure in the pump has to fall low enough to suck the liquid from the low-pressure region. This greatly reduces the pressure range left before the liquid starts to boil.
How is Cavitation Damage Caused?
The vapour bubbles collapse when the pressure in the liquid increases. The implosion occurs in milliseconds and microscopic ‘torpedoes’ of liquid are ejected at up to 500 m/s (1650 ft/sec). Continuous impact on pump internals gradually shred the metal surface. The erosion process is accelerated in corrosive situations as the jet impact removes the corrosion coating and exposes fresh metal to the liquid and corrosion accelerates.
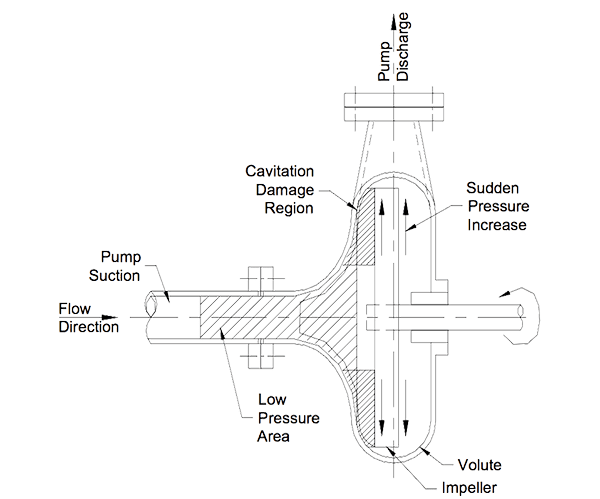
The whip cracking sounds heard when cavitation occurs is a result of the impact from the opposing walls of water in collapsing bubbles. An impeller suffering severe cavitation is full of ragged edged holes and looks like worms have attacked it. Early signs of cavitation show up as pitted and roughened patches in localised areas just down stream of the low-pressure areas.
Entrained Gases Make the Problem Worse
Gas dissolved in a liquid will come out of solution well before the vapour pressure (cavitation pressure) of the liquid is reached. The bubbles rising in a glass of beer is dissolved gas (carbon dioxide) coming out of solution – no cavitation is present but gas bubbles have formed. Increasing the pressure will again dissolve the bubbles. If this were to happen in a pump, bubbles would appear at the low-pressure regions and then dissolve in the high-pressure region. All the while the bubbles are moving with the liquid.
The undissolved gas bubbles can act as nucleation sites for vapour bubbles. The amount of cavitation in situations containing dissolved gasses is greatly increased and it spans a greater range of pressures and temperatures. If dissolved gasses are present it is even more critical to keep pressure loss to a minimum and temperatures cool.
What Can Be Done to Reduce Cavitation?
To stop or reduce cavitation we need to maintain pressure above vapour pressure. This can be achieved by pressurising the suction line, by cooling the liquid and by reducing the friction losses. Because the velocity directly affects pressure the first thing that can be done is to close off a valve on the discharge of the pump and slow the liquid down. Another way to raise the pressure on the suction side is to raise the liquid level at the source.
Often cavitation is created at the engineer’s drawing board. Small bore pipes and oversized impellers cause greater flow velocities than necessary. The high velocity will cause high friction, which produces high-pressure loss resulting in cavitation. Size the impeller to give a flow velocity in the suction pipe of 1 to 2.5 meters per second (3 to 8 feet per second) or increase the size of the suction pipe if a large pump is required.
Where negative pressures (vacuum) cannot be avoided they should be prevented from exceeding about two-third the difference between atmospheric pressure and the vapour pressure.
Suction piping design is more important than the discharge piping design. Use long radius elbows, use full-bore valves, make smooth changes to cross-sections, keep the pump as far below the source as possible, keep branches at least 10 pipe diameters up stream of the suction flange and have easy access to strainer screens for operators to clean.
Provided the pump produces more pressure than necessary, machining the impeller diameter down can reduce the velocity. Check our article on “Changing the Service Duty of a Pump” for the formulas needed to determine the new impeller size.
Adding inducers to the impeller can reduce pipe friction. An inducer is a large pitch screw that fits down the suction pipe and draws the liquid into the pump. They can reduce pressure loss by up to 50% of that which would have occurred.
Mike Sondalini – Maintenance Engineer
If you found this interesting, you may like the ebook Centrifugal Pump Problems & Answers.
 Ask a question or send along a comment.
Please login to view and use the contact form.
Ask a question or send along a comment.
Please login to view and use the contact form.
I appreciate it that you discussed how maintaining pressure above vapor level could help reduce cavitation in a centrifugal pump. Should Dad have to keep his centrifugal pump working properly, I suppose he’d have to prevent bubbles from forming inside it to minimize air for conflating interior pressure in the pump. He could very well achieve this by bolstering the pressure outside of the suction side and increasing the level of water inside it.